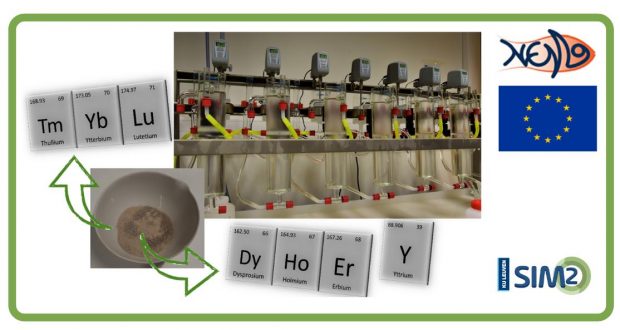
… is an open, quadruple helix network that supports the required innovation with respect to ...
Read More »Europe has somewhere between 150,000 and 500,000 landfill sites, with an estimated 90% of them being “non-sanitary” landfills, predating the EU Landfill Directive of 1999. These older landfills tend to be filled with municipal solid waste and often lack any environmental protection technology. In order to avoid future environmental and health problems, many of these landfills will soon require expensive remediation measures. This situation might appear bleak, but it does present us with an exciting opportunity for a combined resource-recovery and remediation strategy, which will drastically reduce future remediation costs, reclaim valuable land, while at the same time unlocking valuable resources. However, the widespread adoption of Enhanced Landfill Mining (ELFM) in the EU, as envisaged by NEW-MINE, urgently requires skilled scientists, engineers, economists and policy makers who can develop cost-effective, environmentally friendly ELFM practices and regulatory frameworks. All this demands a European commitment to concerted, inter- and transdisciplinary research and innovation.
Read more...
EURELCO
News
-
Join us at ICHS 2024 Symposium (9-11 Sep 2024, Mechelen)
Full programme for ICHS 2024 is out (First International Symposium on Circular Hydrometallurgy, Mechelen, Belgium, 9-11 September 2024).
Read More »
Events
-
Seventh NEW-MINE network-wide event and closing symposium in Leuven (Belgium; Febr. 2020)
The seventh network-wide event of MSCA ETN NEW-MINE took place on 5, 6 and 7 ...
Read More »
In the press
-
NEW-MINE project featured in Recycling Magazine
On July 13, 2020, STADLER, one of the NEW-MINE partners, launched a press release about ...
Read More »